Welcome to the second edition of my weekly feature Photonics Briefs. This week I’m going to go a bit more in depth on attosecond wavefunction imaging and highlight an article on terahertz near-field imaging.
First, in the online prepublication of Nature Photonics, there’s an article discussing on-chip near-field terahertz imaging. There’s a lot of buzz words there, so let’s figure out what’s going on.
Author’s Kawano and Ishibashi report the development of a semiconductor chip (like the processor in your computer) which features an integrated terahertz aperture, probe, and detector. The advantage is that with all of this integrated into one casing you firstly save space (and eventually cost), but also gain the ability to do high-resolution near-field imaging.
Typically one cannot take an image of an object that is smaller than the wavelength of the light being used. For example, you can’t use your digital camera to get a picture of something that’s half a micron (10-6 scanning tunnelling microscope which uses a sharp point to generate maps of surfaces to around a nanometre accuracy.
So what’s cool about Kawano’s paper is that they’ve created a single chip which can generate Thz images with sub-milimeter resolution. Remember that THz-imaging is unique in that it can transmit through things opaque to visible light (like cloth, plastic, etc.) but can still detect metals and some other materials.
- Yukio Kawano & Koji Ishibashi, “An on-chip near-field terahertz probe and detector”, Nature Photonics ADVANCED ONLINE PUBLICATIONS, 10 August 2008.
Now, to go a bit more in depth on Paul Corkum group’s paper on imaging the wavefunction of Nitrogen.
Basically, the wavefunction (or orbital to chemists) is a mathematical construct. That is, it’s something physicists made up to fit the data. However, many made up constructs have proven so useful that it is likely they are accurate representations of the real world. The best example of this is the photon – the idea that light is comprised of particles of waves (or wave-packets). When two molecules wavefunctions interact, we have chemistry. Unfortunately this takes place on time scales of attoseconds (10-18 s), which until recently have not been accessible to measure. Recent advances in laser technology have made this possible however.
What Dr. Corkum’s group does is take an ultrafast (femtosecond 10-15 s) laser source, and mix pulses together in such a way that even shorter pulses are created. I can’t fully explain it here, since I don’t fully understand what’s going on in this situation. An analogy can be made when you think of beat frequencies of sound. This happens when you mix two (or more) low frequencies together and can generate higher frequencies (or inversely shorter pulses).
To image the wavefunctions it requires three steps: aligning the molecules relative to the lab, selecting an ionization, and copying the state to a laser pulse.
The first two steps are necessary so that they know what they’re looking at. The method of imaging they use is “computed tomography” which takes one-dimensional projections of an image and creates cross-sections and generates a 3-D image from the information. This requires an object that can be fixed in a reference frame, and rotated. This is apparently not as big of a deal for lasers to do with single atoms, so steps one and two are essentially done.
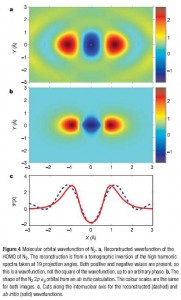 They then use the attosecond pulses to image slices of the atom – which yields the frequency information about the highest occupied molecular orbital (the valence band, or where all the chemistry occurs) of the molecule. Their process also retains the phase of the wave going through the molecule – which is key to generating the absolute wavefunction, and not just the probability cloud (or the wavefunction squared, which is what is usually detected – this shows where the electrons are “most likely to be”).
They then use the attosecond pulses to image slices of the atom – which yields the frequency information about the highest occupied molecular orbital (the valence band, or where all the chemistry occurs) of the molecule. Their process also retains the phase of the wave going through the molecule – which is key to generating the absolute wavefunction, and not just the probability cloud (or the wavefunction squared, which is what is usually detected – this shows where the electrons are “most likely to be”).
If this hasn’t blown your mind yet (it should), they finish the paper by proposing that they should theoretically be able to watch electrons move. What they suggest is that they will be able to watch chemistry happen, i.e. watch a molecule interact with another.
- J. Itantani, J. Levesque, D. Zeidler, Hiromichi Niikura, H. Pepin, J. C. Kieffer, P. B. Corkum & D. M. Villeneuve, “Tomographic imaging of molecular orbitals”, Nature 432, 867-871 (2004).
This ends edition 2 of Photonics Briefs
[tags]photonics, lasers, wavefunctions, quantum mechanics, semiconductors, terahertz[/tags]